Vaccines are among our greatest public health achievements, having helped eradicate or control debilitating infectious diseases including smallpox, polio, measles, and Ebola. Yet new diseases continue to emerge, as evidenced by COVID-19, along with new strains of existing viruses. Vaccine research and development remain vital to the health of our country and our world, emphasizing the vital importance of vaccine trials.
Vaccines prevent rather than treat existing conditions, meaning they save vast amounts of money in healthcare costs. In addition to the financial savings of a vaccine, they also save lives from stress caused by illness, long-term effects, and mental health issues exacerbated by disease. The Infectious Diseases Society of America states every dollar spent on childhood vaccines saves an estimated 10+ dollars in health care expenses. The CDC estimates that vaccination of children born between 1994 and 2021 in the U.S. will prevent 472 million illnesses, helping to avoid over 1 million deaths, and saving nearly $2.2 trillion in total societal costs (including $479 billion in direct healthcare costs). According to this data, vaccines are evidently one of public health’s most cost-effective tools.
Following the success of mRNA vaccines developed for COVID-19, there will potentially be other breakthroughs for other viruses and conditions, including influenza, herpes, and even cancer. At Aim Trials, we are ready to take on the challenge and increasing need for vaccine trials. Our facility is equipped for these studies, and our team is trained and ready to promote and conduct research on new vaccines.
Vaccine Trials Drive Safety and Efficacy
The World Health Organization defines a vaccine’s efficacy measurement as “based on how many people who got vaccinated developed the ‘outcome of interest’ (usually disease) compared with how many people who got the placebo developed the same outcome.” This measurement is done through controlled, stringent clinical trials, including large Phase 3 efficacy studies, to establish safety and benefit before approval and availability. Thanks to strict federal oversight and institutional review boards, these trials adhere to high ethical standards with regular reporting.
The Future of Vaccine Innovation
Dozens of new vaccines are in development globally, leveraging advances in immunology and molecular biology to target pathogens like HIV, Zika, and respiratory syncytial virus (RSV). Exciting new platforms can enable faster production against emerging outbreaks. The Food and Drug Administration recently gave fast-track status to a vaccine to treat the effects of Alzheimer’s disease.
In 2023, the United States government revealed plans for Project NextGen, a $5 billion initiative to create improved COVID-19 vaccines. Project NextGen aims to produce nasal vaccines that may more effectively prevent viral infection. The project also seeks to develop vaccines that could potentially work against multiple types of coronaviruses, not just the one that causes COVID-19. By investing in next-generation vaccine technologies like these, the government hopes to stay ahead of future coronavirus threats.
Realizing these innovations in vaccines requires diligent, ethical clinical trials. As public health threats multiply, organizations like Aim Trials will remain instrumental partner with pharmaceutical companies and our government in proving the efficacy and safety of new vaccines.
Why Aim Trials for Vaccine Research?
Aim Trials has successfully partnered on Phase 2-4 trials for a multitude of vaccines including RSV, influenza, COVID-19, meningococcal and more.
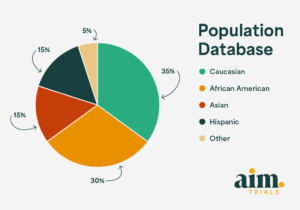
As a multi-specialty site integrated within a private practice, Aim Trials has extensive experience conducting high-volume vaccine studies. For the RSVpreF trial, we achieved a 98% eCOA compliance rate. Our population database includes over 20,000 patients, with a diverse makeup of 35% Caucasian, 30% African American, 15% Asian, 15% Hispanic, and 5% other minorities. This patient mix ensures we can recruit effectively across hard-to-reach demographics to quicky and safely help with most studies. Our team has exceptional retention and compliance rates. Aim Trials prides itself on providing excellence in trial execution and unrelenting participant protections, helping to drive the field forward with the goal of saving lives through science.
Contact [email protected] to learn about our vaccine trial capabilities.